
Randomized controlled trial in insulin-naive people with T2DM using A1C as the primary endpoint1


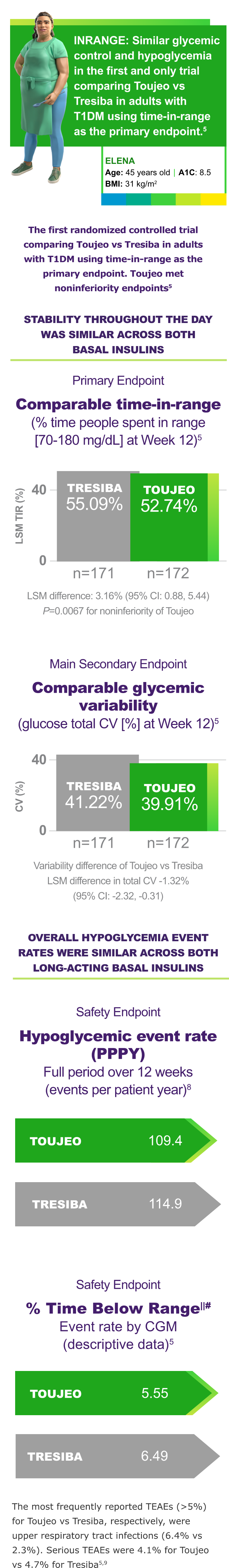
First randomized controlled trial in people with T1DM using Time-in-Range as the primary endpoint5

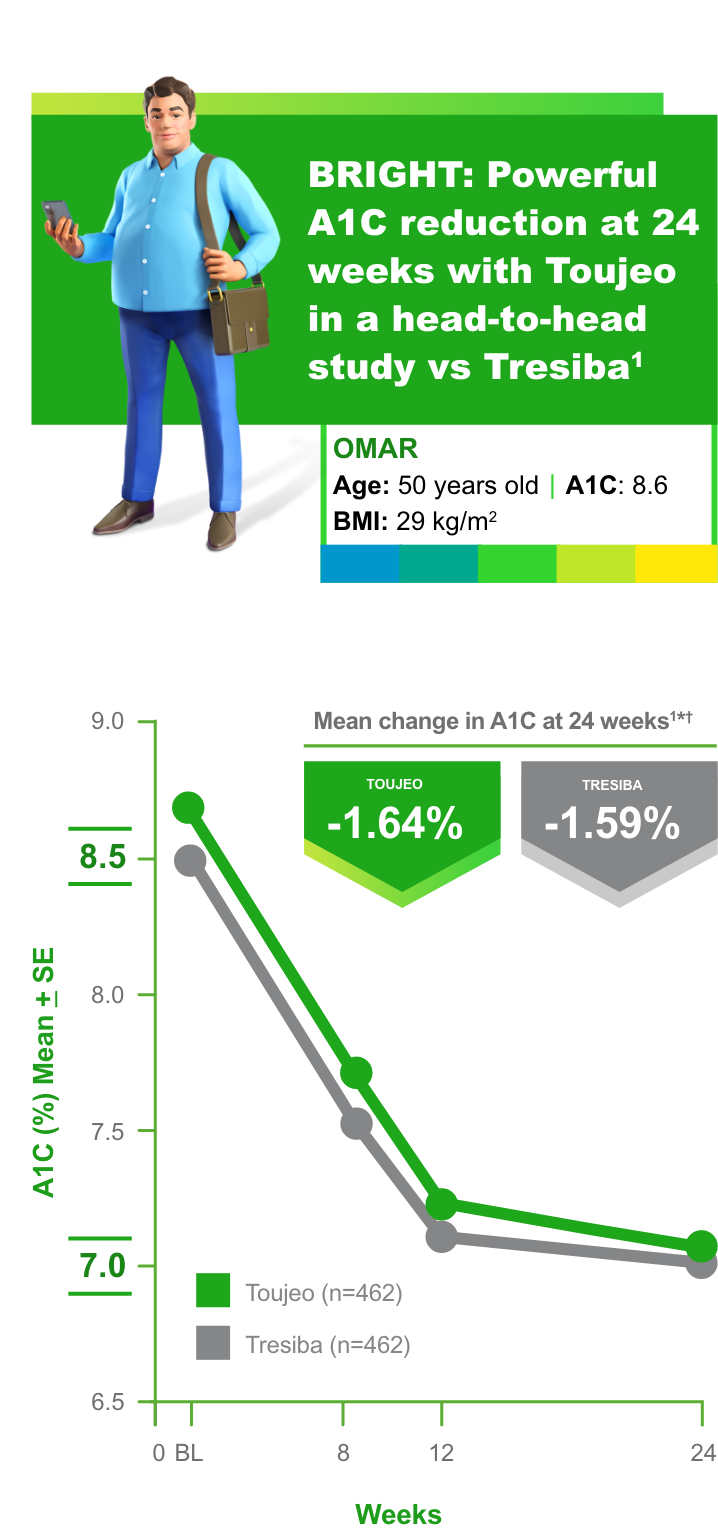
*Baseline: 8.72% ± 0.83% for Toujeo, 8.57% ± 0.80% for Tresiba.1
†At 24 weeks: 7.03% ± 0.79% for Toujeo, 7.03% ± 0.77% for Tresiba.1
BRIGHT study Toujeo vs Tresiba: Primary endpoint achieved
- Noninferiority margin of 0.3% and difference between treatments of -0.05% (95% confidence interval -0.15 to 0.05) at 24 weeks1
- The mean daily basal insulin dose on Day 1 was higher in the Toujeo group than in the Tresiba group: 16.86 Units and 10.15 Units, respectively. By Week 24, the mean daily basal insulin dose was 50.47 Units and 39.21 Units for Toujeo and Tresiba, respectively2
- Mean (±SD) body weight increased from baseline (90.6 ± 16.1 and 88.7 ± 15.9 kg with Toujeo and Tresiba, respectively) to Week 24 (92.5 ± 16.6 kg and 91.4 ± 16.7 kg), an absolute mean increase of 2.0 ± 3.8 kg with Toujeo and 2.3 ± 3.6 kg with Tresiba1
The most frequently reported TEAEs (≥5%) were viral upper respiratory tract infection reported in 8.2% in the Toujeo group and in 8.7% in the Tresiba group, and upper respiratory tract infection in 5.2% and 4.1%, respectively. Serious TEAEs were 4.5% for Toujeo vs 4.3% for
- This trial was not designed to evaluate the relative safety of Toujeo compared with Tresiba, and comparator adverse event rates are not an adequate basis for comparison rates between the products
- Hypoglycemia events can vary among studies based on study design, method of collecting data, and hypoglycemia definitions. Hypoglycemia is the most frequently reported adverse event for all insulins. Therefore, each patient should be evaluated to determine individual risk and how to recognize hypoglycemic signs and symptoms and the actions to be taken should they occur3


Hypoglycemia
(Events per patient year over 24 weeks)1‡
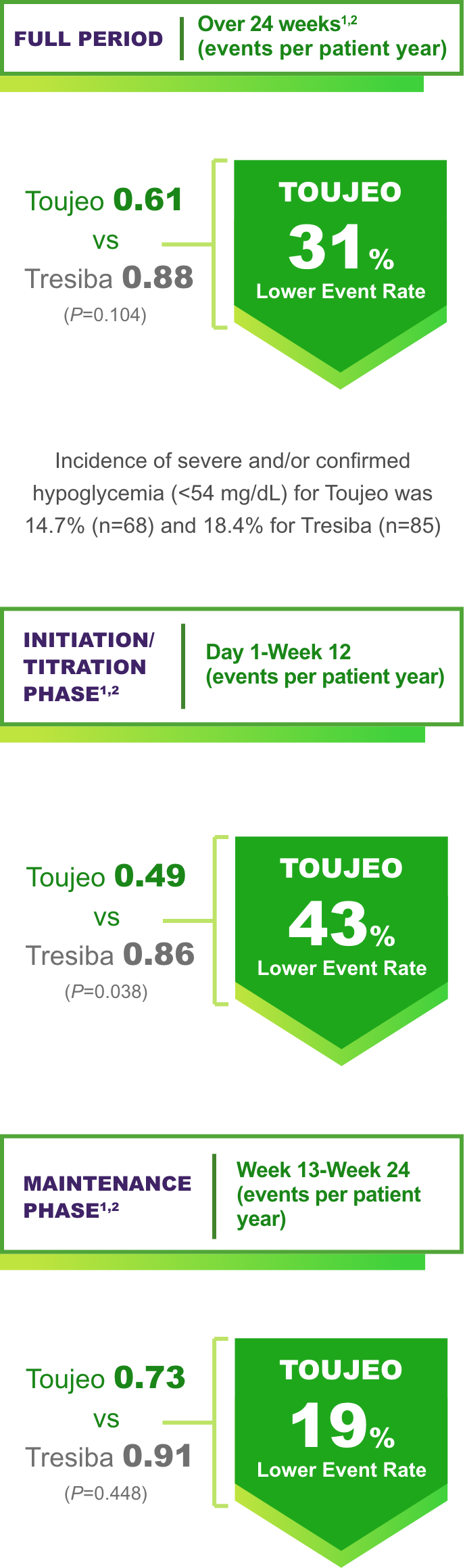
P values are for descriptive purposes only and are not adjusted for multiplicity.
Event rates during Maintenance Phase were comparable.
Incidence of severe and/or confirmed hypoglycemia was 7.8% for Toujeo (n=36) vs 11.7% for Tresiba (n=54) in the Titration Phase.2
Incidence of severe and/or confirmed hypoglycemia (<54 mg/dL) for Toujeo was 14.7% (n=68) and 18.4% for Tresiba (n=85).2
Incidence of severe and/or confirmed hypoglycemia was 9.8% for Toujeo (n=44) vs 11.2% for Tresiba (n=50) in the Maintenance Phase.2
‡Anytime severe and/or confirmed hypoglycemia (<54 mg/dL).1
Hypoglycemia is the most common adverse event associated with insulin-containing therapies.


BRIGHT renal subgroup data: Greater A1C reduction observed with Toujeo in patients with renal insufficiency6,7
Renal function subgroups (eGFR ≥90 [normal], 60 to <90 [mild] and <60 mL/min/1.73 m2 [moderate/severe])7
A1C reduction was consistent with the BRIGHT primary endpoint7
Comparable risk of hypoglycemia7
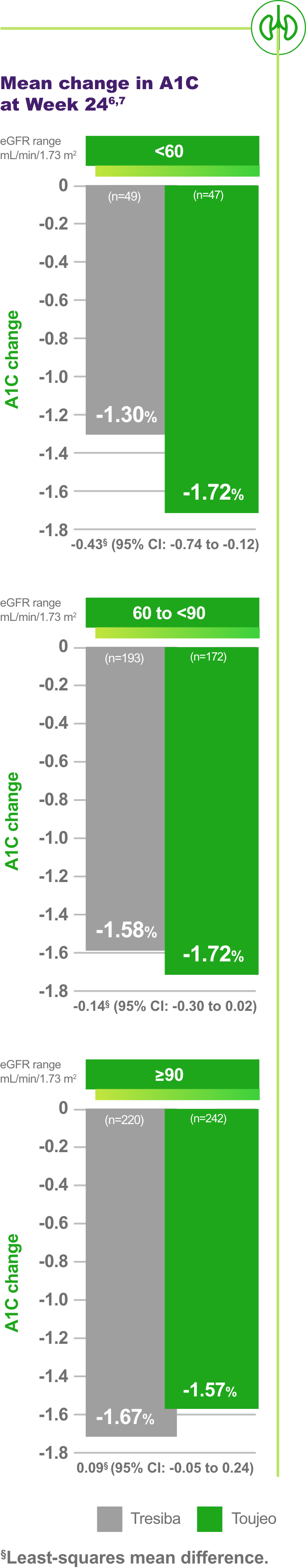
BRIGHT study Toujeo vs Tresiba: Subgroup analysis7
Anytime (24h) confirmed (≤70 mg/dL) hypoglycemia events per patient year:
- Toujeo 6.5 vs Tresiba 10.5
(≥90 mL/min/1.73 m2) - Toujeo 12.3 vs Tresiba 10.5
(60 to <90 mL/min/1.73 m2) - Toujeo 13.5 vs Tresiba 13.9
(<60 mL/min/1.73 m2)
Limitation: This exploratory subgroup analysis was not designed or powered to detect differences between treatment groups. The effect of renal impairment on the PK of Toujeo has not been studied. Frequent glucose monitoring and dose adjustment may be necessary.3
A multicenter, open-label, randomized, active-controlled, two-arm, parallel-group, noninferiority study comparing the efficacy and safety of Toujeo and Tresiba 100 Units/mL in adult insulin-naive patients with T2DM not adequately controlled with OADs ± a GLP-1 receptor agonist. Patients were randomized 1:1 to receive Toujeo (n=466) or Tresiba (n=463) subcutaneously between 6 PM and 8 PM over 24 weeks. The starting doses of Toujeo and Tresiba were 0.2 Units/kg and 10 Units once daily, respectively, in accordance with product label and the same insulin titration algorithm was followed. Investigational medicinal products were injected in the evening between 6 PM and 8 PM. Doses were adjusted at least weekly, but not more often than every 3 days, targeting a fasting SMPG of 80 to 100 mg/dL while avoiding hypoglycemia. The aim of the titration period (Day 1 to Week 12) was the achievement of the fasting SMPG target. The primary endpoint of this study was to demonstrate the noninferiority of Toujeo to Tresiba in A1C change from baseline to Week 24. Limitations: Open-label and short duration, 24 weeks1,2

Hypoglycemia is the most common adverse event associated with insulin-containing therapies
Primary endpoint: LSM difference 3.16% (95% CI: 0.88, 5.44), P=0.00675¶
- A hierarchical step-down testing procedure was applied to the primary efficacy endpoint and main secondary endpoints: noninferiority for glucose total coefficient of variation, and superiority for percentage TIR, to control for type I error. Superiority of Toujeo with respect to Tresiba was not demonstrated on the primary efficacy endpoint (LSM difference, -2.35% [-4.75 to 0.05]; P=0.0548)5,10
llAny documented or severe hypoglycemia.5,10
¶Noninferiority was assessed in the intent-to-treat population. Noninferiority of the primary endpoint was demonstrated if the lower bound of the two-sided 95% CI of the adjusted difference estimate for m1–0.9 m0 (where m1 and m0 are the true means for Toujeo and Tresiba groups, respectively) was greater than 0.10
#Time below range is defined as % time people spent <70 mg/dL.5
A multicenter, randomized, active-controlled, parallel-group, 12-week, open-label, Phase 4 noninferiority study in adults with T1DM not adequately controlled with basal and mealtime insulin. Patients were randomized 1:1 to receive Toujeo (n=172) or Tresiba 100 units/mL (n=171) once daily in the morning. The primary endpoint was noninferiority of Toujeo vs Tresiba in percentage of time spent in range (70-180 mg/dL) at Week 12. Limitations: Open label, 24 weekduration.5,10


Order samples today†
Have samples delivered to your office.
†US-licensed prescribers are required to register to request samples.‡

Toujeo added to GLP-1 RA: A real-world data study
See the effect on A1C and hypoglycemia after Toujeo was added to a GLP-1 RA in insulin-naive patients with T2DM.
Indication
Toujeo is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients 6 years and older with diabetes mellitus. Limitations of Use: Toujeo is not recommended for the treatment of diabetic ketoacidosis.
Important Safety Information
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
A1C, glycated hemoglobin; CGM, continuous glucose monitoring; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide-1; LSM, least squares mean; OAD, oral antidiabetic drug; PK, pharmacokinetics; PPPY, per patient, per year; SD, standard deviation; SE, standard error; SMPG, self-monitored plasma glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TEAE, treatment-emergent adverse event; TIR, time in range.
‡ OHIO HCPs: Prescription drug samples may only be provided to a prescriber whose practice is licensed as a Terminal Distributor of Dangerous Drugs ("TDDD") or qualifies as exempt under Ohio law. For more information on the State of Ohio Board of Pharmacy Prescriber licensure requirements, how to obtain a TDDD license, and reasons for exemptions, please go to: http://www.pharmacy.ohio.gov/Licensing/TDDD.aspx
References:
1. Rosenstock J, et al. Diabetes Care. 2018;41(10):2147-2154. doi:10.2337/dc18-0559 2. Data on file. Clinical Study Report, LPS14584. Sanofi US 2018. 3. Toujeo Prescribing Information. 4. Bolli GB, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386-394. doi: 10.1111/dom.12438 5. Battelino T, et al. Time in range using insulin glargine 300 U/mL versus insulin degludec 100 U/mL in type 1 diabetes: head-to-head randomised controlled InRange trial. Abstract LB009/#1015. Presented at Advanced Technologies & Treatments for Diabetes. April 27-30, 2022. Barcelona, Spain. 6. Data on file. Clinical Study Report, LPS14584. CSR BRIGHT HBA1C. Sanofi US 2018. 7. Haluzík M, et al. Diabetes Obes Metab. 2020;22(8):1369-1377. doi:10.1111/dom.14043 8. Data on file. Clinical Study Report, LPS14947. Key InRange. Sanofi US 2022. 9. Data on file. Clinical Study Report. DOF InRange. Sanofi May 2019. 10. Battelino T, et al. Diabetes Ther. 2020;11(4):1017-1027. doi:10.1007/s13300-020-00781-6
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
A1C, glycated hemoglobin; CGM, continuous glucose monitoring; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide-1; LSM, least squares mean; OAD, oral antidiabetic drug; PK, pharmacokinetics; PPPY, per patient, per year; SD, standard deviation; SE, standard error; SMPG, self-monitored plasma glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TEAE, treatment-emergent adverse event; TIR, time in range.
‡ OHIO HCPs: Prescription drug samples may only be provided to a prescriber whose practice is licensed as a Terminal Distributor of Dangerous Drugs ("TDDD") or qualifies as exempt under Ohio law. For more information on the State of Ohio Board of Pharmacy Prescriber licensure requirements, how to obtain a TDDD license, and reasons for exemptions, please go to: http://www.pharmacy.ohio.gov/Licensing/TDDD.aspx
References:
1. Rosenstock J, et al. Diabetes Care. 2018;41(10):2147-2154. doi:10.2337/dc18-0559 2. Data on file. Clinical Study Report, LPS14584. Sanofi US 2018. 3. Toujeo Prescribing Information. 4. Bolli GB, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386-394. doi: 10.1111/dom.12438 5. Battelino T, et al. Time in range using insulin glargine 300 U/mL versus insulin degludec 100 U/mL in type 1 diabetes: head-to-head randomised controlled InRange trial. Abstract LB009/#1015. Presented at Advanced Technologies & Treatments for Diabetes. April 27-30, 2022. Barcelona, Spain. 6. Data on file. Clinical Study Report, LPS14584. CSR BRIGHT HBA1C. Sanofi US 2018. 7. Haluzík M, et al. Diabetes Obes Metab. 2020;22(8):1369-1377. doi:10.1111/dom.14043 8. Data on file. Clinical Study Report, LPS14947. Key InRange. Sanofi US 2022. 9. Data on file. Clinical Study Report. DOF InRange. Sanofi May 2019. 10. Battelino T, et al. Diabetes Ther. 2020;11(4):1017-1027. doi:10.1007/s13300-020-00781-6


