In a PK/PD study: A longer-lasting, glucose-lowering effect vs Lantus®1,2
These PK/PD data do not support a comparison of the safety or efficacy of Toujeo and Lantus.
Time course effect following product administration (N=30)1,2
Once-daily Toujeo® should be injected at the same time each day.

These PK/PD data do not support a comparison of the safety or efficacy of Toujeo and Lantus. 1,2
Clamp Study 1:
The PDs of Toujeo at steady state after 8 days of daily injections were evaluated against Lantus in a euglycemic clamp study of patients with T1DM (N=30) receiving injections of 0.4 U/kg once daily. The dose on Day 8 was followed by a 36-hour euglycemic clamp.
CLAMP Study Limitations: Difficulty in extrapolating results directly to clinical practice due to the experimental clamp setting.1


In pivotal trials, consistent A1C reduction and demonstrated safety profile in a broad range of adult patients2-6
Click on a tab to see efficacy and safety results for each of the EDITION clinical trials.
-
T2DM: Insulin-naive and previously on OADs3

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T2DM were nasopharyngitis (7.1%) and upper respiratory tract infection (5.7%).2
- Incidence of severe hypoglycemia when part of a multiple-dose injection regimen: 6.6% in T1DM, 5% in T2DM; when part of a basal insulin-only regimen: 1.0% and 0.9% in two T2DM studies2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
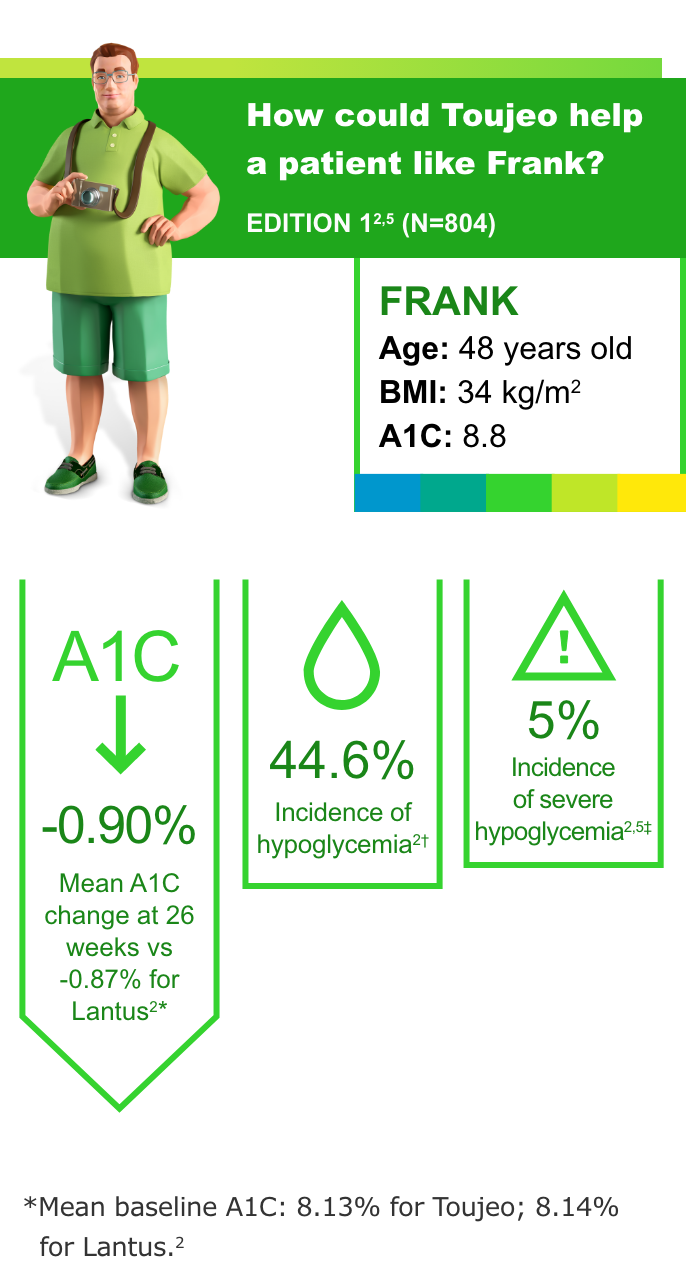
T2DM: Insulin-naive and previously on OADs3

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T2DM were nasopharyngitis (7.1%) and upper respiratory tract infection (5.7%).2
- Incidence of severe hypoglycemia when part of a multiple-dose injection regimen: 6.6% in T1DM, 5% in T2DM; when part of a basal insulin-only regimen: 1.0% and 0.9% in two T2DM studies2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
-
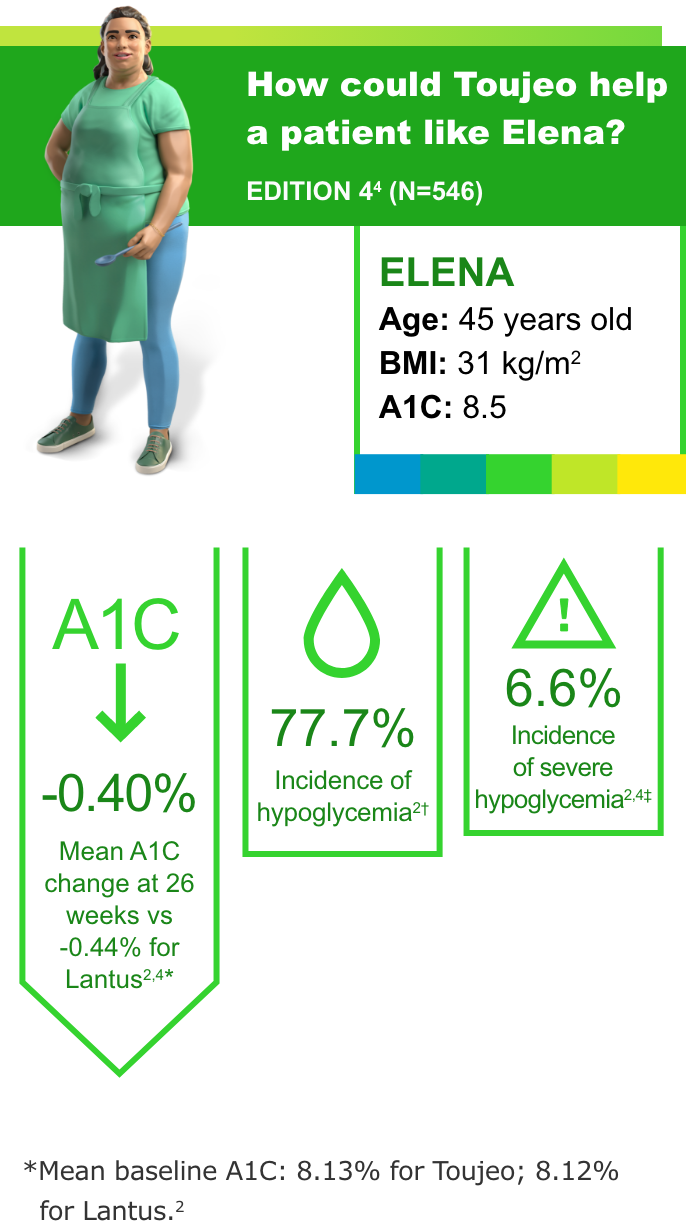
T1DM: Previously on basal and mealtime insulin4

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T1DM were nasopharyngitis (12.8%) and upper respiratory tract infection (9.5%).2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
T1DM: Previously on basal and mealtime insulin4

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T1DM were nasopharyngitis (12.8%) and upper respiratory tract infection (9.5%).2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
-
T2DM: Previously on basal and mealtime insulin5

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T2DM were nasopharyngitis (7.1%) and upper respiratory tract infection (5.7%).2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
T2DM: Previously on basal and mealtime insulin5

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T2DM were nasopharyngitis (7.1%) and upper respiratory tract infection (5.7%).2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
-
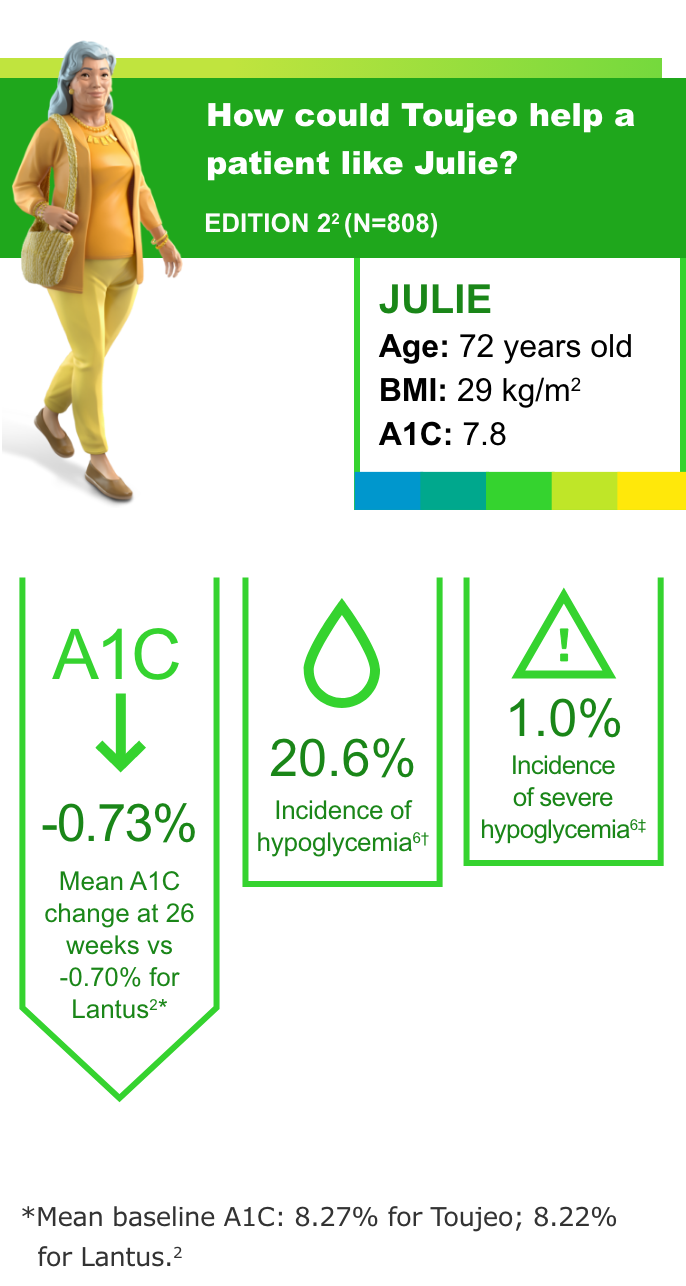
T2DM: Previously on basal insulin and OADs6

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T2DM were nasopharyngitis (7.1%) and upper respiratory tract infection (5.7%).2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
T2DM: Previously on basal insulin and OADs6

Most common adverse events (with incidence ≥5%) with Toujeo in patients with T2DM were nasopharyngitis (7.1%) and upper respiratory tract infection (5.7%).2
- In all the EDITION studies, Toujeo met the primary endpoint (prespecified noninferiority margin of 0.4% and the 95% CI) at 26 weeks2
- In clinical trials, patients on Toujeo used more basal insulin than patients on Lantus (11%-15% in T2DM; 17.5% in T1DM)2
- To minimize the risk of hypoglycemia, titrate the dose of Toujeo no more frequently than every 3 to 4 days2
- Hypoglycemia is the most common adverse event associated with insulin-containing therapies
There were no clinically important differences in body weight between treatment groups.2
†Documented symptomatic hypoglycemia <3.0 mmol/L (<54 mg/dL).3
‡Severe hypoglycemia: an event requiring assistance of another person to actively administer a resuscitative action.2
All pivotal studies were 26-week, open-label, controlled, titrate-to-target, noninferiority studies in adults with diabetes not at A1C goal (range: 7%-10% or 11%) randomized to Toujeo or Lantus once daily. All patients were titrated to an FPG goal of 80 to 100 mg/dL (T2DM) or 80 to 130 mg/dL (T1DM).2-6


See the Toujeo vs Tresiba® studies
See how Toujeo did in head-to-head studies vs Tresiba.

Interactive Dosing Calculator for T2DM patients
Calculate the number of units (including titration estimate), mLs, and boxes to include on 30- or 90-day prescriptions. Writing this information on a prescription may reduce rejections at the pharmacy.
Indication
Toujeo is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients 6 years and older with diabetes mellitus. Limitations of Use: Toujeo is not recommended for the treatment of diabetic ketoacidosis.
Important Safety Information
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
A1C, glycated hemoglobin; FPG, fasting plasma glucose; GIR, glucose infusion rate; OAD, oral antidiabetic drug; PD, pharmacodynamics; PK, pharmacokinetics; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
References:
1. Becker RHA, et al. Diabetes Care. 2015;38(4):637-643. doi:10.2337/dc14-0006 2. Toujeo Prescribing Information. 3. Bolli GB, et al. Diabetes Obes Metab. 2015;17(4):386-394. doi:10.1111/dom.12438 4. Home PD, et al. Diabetes Care. 2015;38(12):2217-2225. doi:10.2337/dc15-0249 5. Riddle MC, et al. Diabetes Care. 2014;37(10):2755-2762. doi:10.2337/dc14-0991 6. Yki-Järvinen H, et al. Diabetes Care. 2014;37(12):3235-3243. doi:10.2337/dc14-0990
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
A1C, glycated hemoglobin; FPG, fasting plasma glucose; GIR, glucose infusion rate; OAD, oral antidiabetic drug; PD, pharmacodynamics; PK, pharmacokinetics; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
References:
1. Becker RHA, et al. Diabetes Care. 2015;38(4):637-643. doi:10.2337/dc14-0006 2. Toujeo Prescribing Information. 3. Bolli GB, et al. Diabetes Obes Metab. 2015;17(4):386-394. doi:10.1111/dom.12438 4. Home PD, et al. Diabetes Care. 2015;38(12):2217-2225. doi:10.2337/dc15-0249 5. Riddle MC, et al. Diabetes Care. 2014;37(10):2755-2762. doi:10.2337/dc14-0991 6. Yki-Järvinen H, et al. Diabetes Care. 2014;37(12):3235-3243. doi:10.2337/dc14-0990