Reduction in mean A1C1

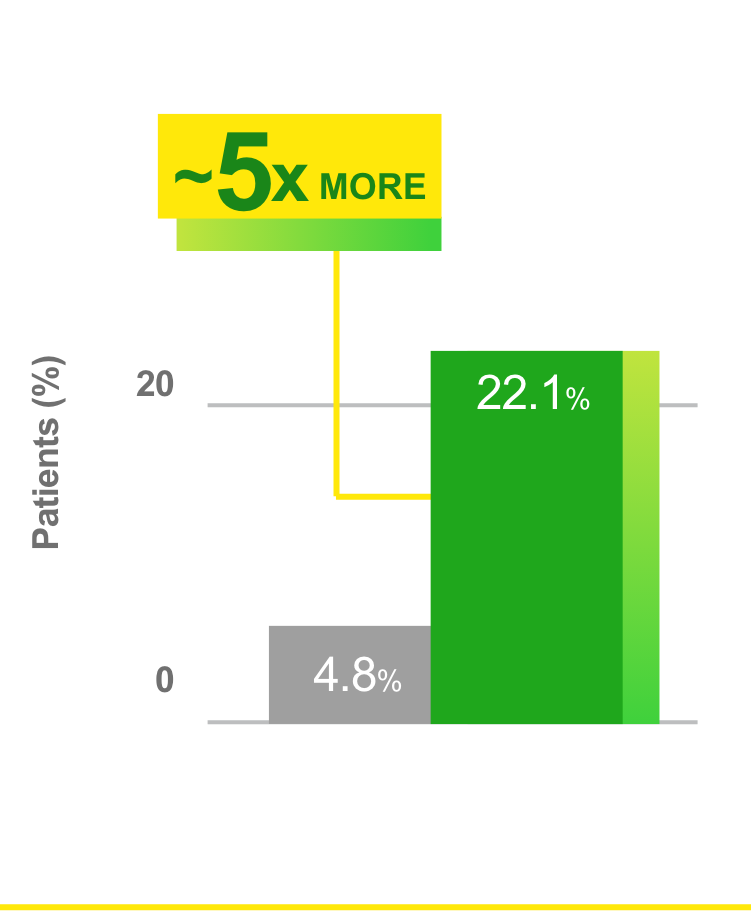
Proportion of patients who achieved A1C goal of <7%1
Overall hypoglycemia event rates were comparable1
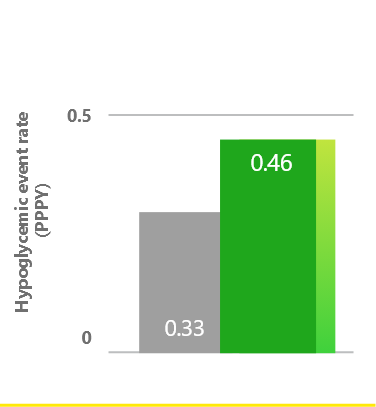
Observed in a retrospective analysis of US EMRs following treatment intensification with Toujeo in adults inadequately controlled on GLP-1 RA ± OAD(s) therapy who had ≥6 months of follow-up data (N=271).1*
Hypoglycemia is the most common adverse event associated with insulin-containing therapies.
DELIVER G pre-post study: A retrospective analysis of US EMRs
Limitations1:
- Results should be interpreted with caution because of the retrospective pre-post design without a control or comparator arm and the relatively short follow-up (6 months)
- Hypoglycemia may have been underreported, as only the clinically significant events were likely to have been captured (eg, no SMBG or CGM data)
- EMR database analysis as a potential for selection bias that cannot be controlled, as well as EMR data-capture challenges (eg, coding errors, no dosing data)
- Patients were mainly from the Northwest and Southern US states and therefore may not be representative of the US national landscape
- Sanofi-sponsored study
*Follow-up period ≥6 months included GLP-1 RA + Toujeo (N=271).1

- Adults with a diagnosis of T2DM
- Diagnosis of T2DM
- Receiving GLP-1 RA therapy that was intensified by adding Gla-300 between March 1, 2015 and September 30, 2019
- Insulin–naive prior to treatment intensification
- ≥12 months’ pre-Gla-300 data and ≥6 months of follow-up data available
- HbA1c 3% to 15%, recorded in the 6 months prior to intensification with Gla-300 and 3- to 9-months after intensification
DELIVER G was a retrospective pre-post study of 271 adult patients with type 2 diabetes receiving GLP-1 RA therapy who were insulin-naive prior to subsequent treatment intensification with Gla-300. DELIVER G included data from a large US EMR data source (IBM® Explorys).1
Patient Identification
Eligible Patients1:



Pay no more than $35†
Start with widespread coverage for both commercially insured and Medicare Part D patients.

Interactive Dosing Calculator for T2DM patients
Calculate the number of units (including titration estimate), mLs, and boxes to include on 30- or 90-day prescriptions. Writing this information on a prescription may reduce rejections at the pharmacy.
Indication
Toujeo is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients 6 years and older with diabetes mellitus. Limitations of Use: Toujeo is not recommended for the treatment of diabetic ketoacidosis.
Important Safety Information
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
†Eligibility Restrictions & Offer Terms for Your Patients:
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
A1C, glycated hemoglobin; CGM, continuous glucose monitoring; EMR, electronic medical record; GLP-1, glucagon-like peptide-1; OAD, oral antidiabetic drug; PPPY, per patient per year; RA, receptor agonist; SMBG, self-monitoring of blood glucose; T2DM, type 2 diabetes mellitus.
Reference:
1. Data on file. Clinical Study Report. CS_00375/CEF0057. Sanofi US 2022.
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
†Eligibility Restrictions & Offer Terms for Your Patients:
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
A1C, glycated hemoglobin; CGM, continuous glucose monitoring; EMR, electronic medical record; GLP-1, glucagon-like peptide-1; OAD, oral antidiabetic drug; PPPY, per patient per year; RA, receptor agonist; SMBG, self-monitoring of blood glucose; T2DM, type 2 diabetes mellitus.
Reference:
1. Data on file. Clinical Study Report. CS_00375/CEF0057. Sanofi US 2022.


