
Savings Options

Commercially insured patients pay no more than $35.
Uninsured or commercially insured patients paying cash pay just $35 a month for Toujeo with Insulins Valyou Savings Program.

Widespread Coverage

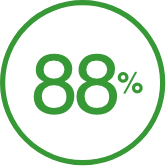
of commercially insured patients are covered†

Medicare patients are covered†
†Formulary data are provided by Managed Markets Insight & Technology, LLC and are current as of 01/2024.


Eligible commercially insured patients pay no more than $35§

§Patients pay no more than $35 for a 30-day supply, depending on insurance coverage. Valid up to 10 packs per fill; Offer valid for one fill per 30-day supply.
If you have any questions about starting your patients right away, please call 855-443-1577.
- Eligible patients using a mail order pharmacy can still use this savings offer if they activate a co-pay card and submit for rebate up to the maximum savings limit. See more at PatientRebateOnline.com or call 866-390-5622.
If you or your office needs additional information, call 855-443-1577 8 AM - 8 PM ET, Monday-Friday (except holidays).
See details below.
‡See Terms and Eligibility Restrictions


It's simple. One set price for uninsured patients or commercially insured patients paying cash

- When using this savings card, prices are guaranteed for 12 consecutive monthly fills for the duration of the program.
- Offer valid for one fill per month. To pay $35 per 30 Day Supply, you must fill all your Sanofi insulin prescriptions at the same time, together each month.
- This card should be used by patients who do not have commercial/private insurance covering their prescription drugs and pay cash for these medications.
- After 12 fills, get a new Insulins Valyou Savings Card by going to www.insulinsvalyou.com or by calling 833-813-0190.
- The Insulins Valyou Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment.
- Offer not valid for SOLIQUA® 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL.
See details below.
‡See Terms and Eligibility Restrictions
Check the formulary status for TOUJEO in your area
The coverage information provided above is intended for reference purposes only.
Please check directly with health plan to confirm coverage.
Powered by: Managed Markets Insight & Technology, LLC. Database current as of December, 2025.
© 2025 Managed Markets Insight & Technology, LLC. All rights reserved.
MAT-US-2403750-v1.0-06/2024


For mail order pharmacies
Eligible patients will receive rebates by mail if co-pay cards are not accepted via mail order pharmacies.

Need help with access support?
Sanofi Patient Connection® can help your office obtain prior authorizations for Toujeo.

Need more resources?
Find helpful materials for you and your patient taking Toujeo.
Indication
Toujeo is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients 6 years and older with diabetes mellitus. Limitations of Use: Toujeo is not recommended for the treatment of diabetic ketoacidosis.
Important Safety Information
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
‡Eligibility Restrictions & Offer Terms for Your Patients:
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Important Safety Information
Contraindications
Toujeo is contraindicated during episodes of hypoglycemia and in patients hypersensitive to insulin glargine or any of the excipients in Toujeo.
Warnings and Precautions
Toujeo contains the same active ingredient, insulin glargine, as Lantus. The concentration of insulin glargine in Toujeo is 300 units per mL (U-300).
Insulin pens and needles must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia. Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Toujeo from another insulin therapy.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Unit for unit, patients started on, or switched to, Toujeo required a higher dose than patients controlled with Lantus. When switching from another basal insulin to Toujeo, patients experienced higher average fasting plasma glucose levels in the first few weeks of therapy until titrated to their individualized fasting plasma glucose targets. Higher doses were required in titrate-to-target studies to achieve glucose control similar to Lantus.
Hypoglycemia is the most common adverse reaction in patients treated with Toujeo and may be life-threatening. The long-acting effect of Toujeo may delay recovery from hypoglycemia compared to shorter-acting insulins.
Medication errors that may lead to hypoglycemia, such as accidental mix-ups between insulin products, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Do not dilute or mix Toujeo with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Toujeo via an insulin pump or intravenously because severe hypoglycemia can occur.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Toujeo, monitor, and treat if indicated.
A reduction in the Toujeo dose may be required in patients with renal or hepatic impairment.
All insulins, including Toujeo, can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dosage adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Toujeo include hypoglycemia, hypersensitivity reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Important Safety Information for Toujeo U-300 (insulin glargine) injection SoloStar and Toujeo Max SoloStar
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying their pen; otherwise, they may not get the correct amount of insulin, which may affect their blood glucose levels. It is especially important to perform a safety test when a patient is using a new pen for the first time.
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
‡Eligibility Restrictions & Offer Terms for Your Patients:
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).


